Determination of microelements in food products
Selenium has been shown to be essential for life and to be toxic at levels little above dose required for health. Dietary level of the desired amount of Se is in the narrow range: consumption of food containing less than 0.1 mg/kg of this element will result in its deficiency, whereas more than 1 mg/kg will led to toxic manifestation. Selenium is a necessary element forming active centres in selenoenzymes by covalent carbon-selenium bounds. The complexity of selenium speciation in living organisms results in different biological availability. Inorganic selenium salts, selenoamino acids and various organoselenium compounds prove powerful cancer chemo-preventive effect. The presence of selenium in supplemented food requires special analytical care in order to control its content in food in inorganic and organic forms to avoid its deficiency or excess in human diet. Little information is still available on the identity of the signals measured for selenium in organic form due to the insufficient sensitivity and selectivity of instrumental methods. The problem can be overcome by preconcentration and purification of selenium species isolated from matrix before the analysis.
For selenium speciation analysis in wheat flour, yeast and eggs, the hyphenation of chromatographic separation with element-specific detection is used. Anion exchange and size exclusion chromatography coupled to ICP MS allows to detect inorganic selenium species and selenomethionine at ppt level.
Preparative size exclusion chromatography is applied for isolation of low molecular compounds and proteins. Purified selenium species are analysed by electrospray MS and structures of isolated compounds are studied following selenium isotopic profile. The isolated fraction containing selenoproteins and Se-containing proteins is treated with trypsin and the product of enzymatic digestion is analyzed by RPLC – ESI MS(/MS). Amino acid sequence of tryptic peptides is reconstructed and compared with proteins sequences in databases.

Determination of arsenic species in drinking water
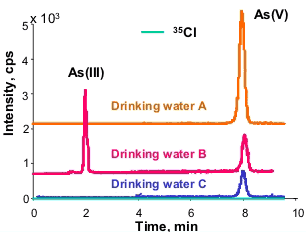
Arsenic can be present in drinking water at least in two different forms: As(III) and As(V). Removal of the excess of highly toxic arsenic(III) is by process of aeration-deironing; during this procedure arsenic is sorbed on the precipitating iron(III) hydroxide. This treatment ensures final concentration of the element in water at the level of 10 ng ml-1.
For arsenic speciation analysis in drinking water, hyphenation of chromatographic separation with element-specific detection is used. The anion exchange chromatography coupled to ICP MS allows to detect inorganic arsenic species at ppt level. The obtained results are verified by determination of total arsenic content in examined products.
Determination of biologically active organic compounds in herbs
The large group of plant polyphenols attracts major interest because of the potential anti-ageing properties presumably based on their functions as natural antioxidants. Flavonoids as a part of them are of particular interest because of their high prevalence in everyday life in fruits, vegetables, spices and herbal remedies. These group of phenolic compounds influence favourably numerous biochemical systems in human organism due to their antioxidant and chelating abilities. They are involved in photosynthesis and cellular energy transfer processes and may serve as precursors of toxic substances. Some flavonoids are known to have pharmacological anti-allergic, anti-inflammatory and anti-viral activity. Flavonoid intake was inversely related to mortality from coronary heart disease among middle-aged people because this group of compounds increase the level of high density lipoprotein cholesterol and inhibit oxidation of low density lipoprotein cholesterol. Flavonoids also suppress cancer cell growth and because of these anti-carcinogenic effects there are the subject of numerous investigations.
The number of flavonoids in plants is large and complex by the occurrence mostly in O-glycosidic forms with a number of sugars such as glucose, galactose, rhamnose, arabidose and rutinose. Glycosilation increase the polarity of the flavonoids and thereby water solubility which is necessary for storage in plant cell vacuoles and optimum intake by human being. In this respect it is speculated that the groups (catechins, anthocyanins, flavones, flavonols, flavanones etc.) differ in their properties depend on number and position of hydroxyl groups and sugar substitutions.

